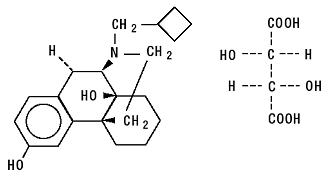
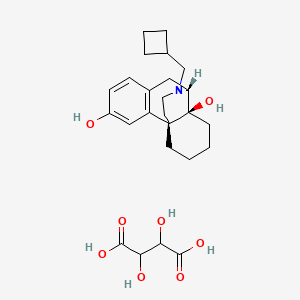
Butorphanol Tartrate Nasal Spray Migraine
Butorphanol tartrate nasal spray migraine. Various codes may apply. If you still have pain after 60-90 minutes your doctor may direct you to use a second spray in the other nostril. If the use is for the treatment of migraine headaches documentation of current prophylactic therapy or documentation of previous trials and therapy failures with two different prophylactic medications must be provided.
This average would require 2 bottles of Stadol per month. CONTRAINDICATIONS BUTORPHANOL butorphanol tartrate Nasal Spray is contraindicated in patients hypersensitive. FDA Approved Indications Butorphanol tartrate nasal solution is indicated for the management of pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
It can be concluded that regimens of butorphanol nasal spray and sumatriptan need not be changed for either pharmacokinetic or safety considerations when the two compounds are co-administered in treating acute migraine. Stadol generic name. Butorphanol has not been linked to serum enzyme elevations during therapy or to clinically apparent liver injury.
It is used for the relief of moderate to severe pain. This average would require 2 bottles of Butorphanol nasal spray per month. BUTORPHANOL butorphanol tartrate Nasal Spray is indicated for the relief of moderate to severe acute pain.
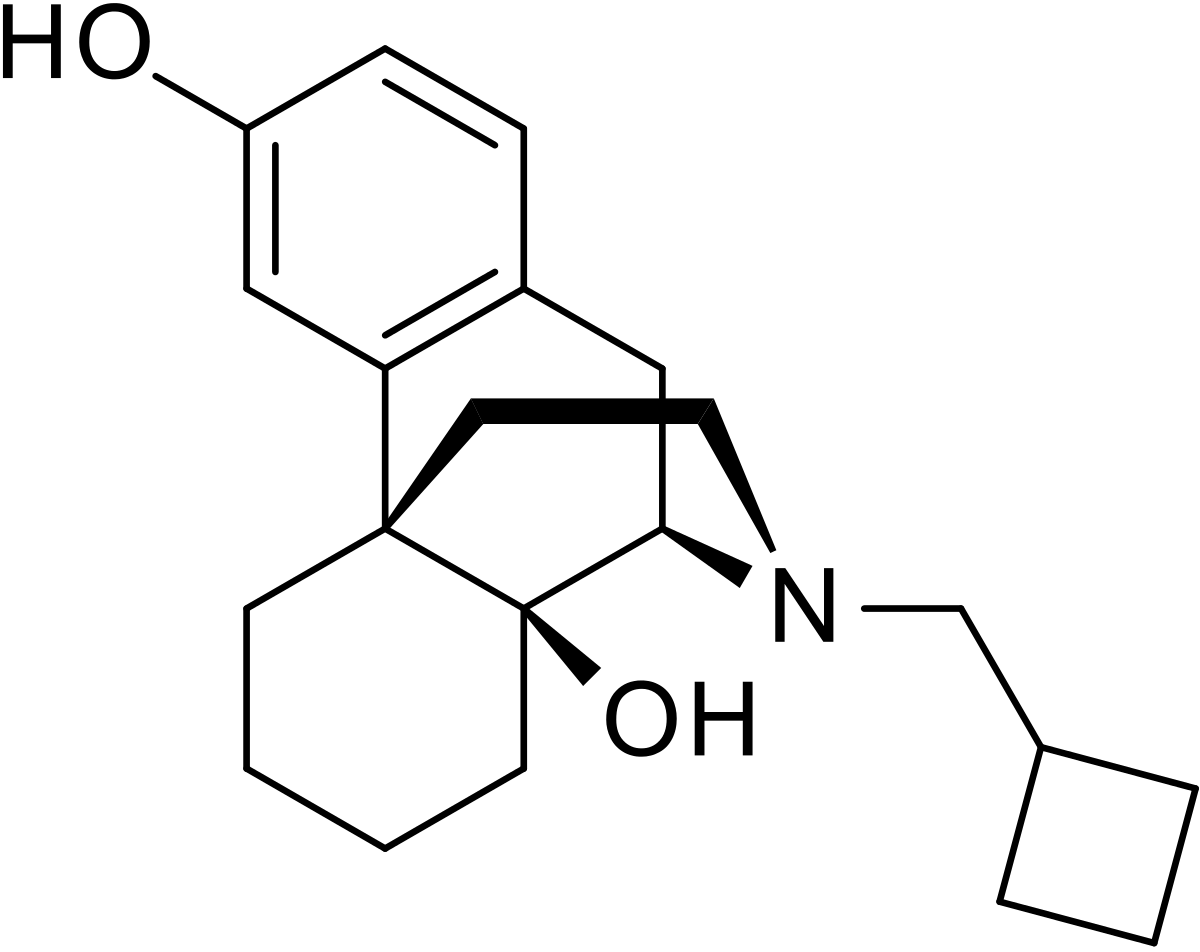
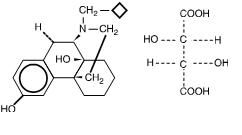
The adverse experience profiles of butorphanol nasal spray were comparable between the treatments with and without sumatriptan. Butorphanol tartrate is a synthetic mixed agonist-antagonist opioid analgesic. Butorphanol has not been linked to serum enzyme elevations during.
For consideration the diagnosis must be supplied. The dose range used to treat a migraine was 2 to 12 mg with 6 mg being average. Stadol is available in intramuscular or intravenous form and most recently as a metered-dose transnasal spray.
Any ICD-9 code that states acute pain from any origin is acceptable. Its transnasal dosage form which may be self-administered when the use of an opioid analgesic is appropriate was previously shown to provide rapid relief of migraine pain.
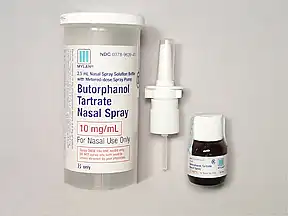
Each bottle of STADOL NS contains 25 mL of a 10 mgmL solution of butorphanol tartrate with sodium chloride citric acid and benzethonium.
Butorphanol nasal spray and sumatriptan were well tolerated. The adverse experience profiles of butorphanol nasal spray were comparable between the treatments with and without sumatriptan. Butorphanol has not been linked to serum enzyme elevations during. FDA Approved Indications Butorphanol tartrate nasal solution is indicated for the management of pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate. Butorphanol is a member of the narcotic analgesics drug class and is commonly used for Anesthesia Anesthetic Adjunct Labor Pain and others. Stadol generic name. If you still have pain after 60-90 minutes your doctor may direct you to use a second spray in the other nostril. The average number of migraine headaches among the population was 4 per month. Butorphanol is an opioid pain reliever similar to morphine.
BUTORPHANOL butorphanol tartrate Nasal Spray is indicated for the relief of moderate to severe acute pain. Ive used Stadol nasal spray with limited success. STADOL NS butorphanol tartrate Nasal Spray STADOL NS is supplied in child-resistant prescription vial containing a metered dose spray pump with protective clip and dust cover a bottle of nasal spray solution and a patient instruction leaflet. The efficacy of BUTORPHANOL Nasal Spray for periods longer than 3 days has not been established. This average would require 2 bottles of Stadol per month. Each bottle of STADOL NS contains 25 mL of a 10 mgmL solution of butorphanol tartrate with sodium chloride citric acid and benzethonium. Stadol is available in intramuscular or intravenous form and most recently as a metered-dose transnasal spray.






















Post a Comment for "Butorphanol Tartrate Nasal Spray Migraine"